Some EpiPen Batches Recalled Due To Potential Failure To Activate
PHILADELPHIA (CBS) – Officials say four batches of EpiPens have been recalled because they may fail to activate or require increased force.
In a statement, Alphapharm said the recall includes about 80,000 EpiPen 300 microgram adrenaline injection auto-injectors. The company says they may contain a defective part and have been distributed worldwide.
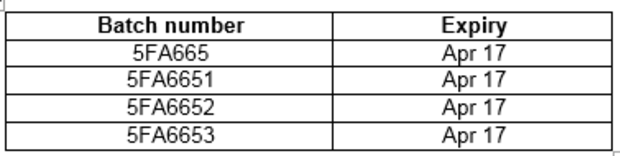
Batches 5FA665, 5FA6651, 5FA6652, 5FA6653 are included in the recall.
Officials say there have been two reports confirming the device failed to activate.
The company says, "The failure of the auto-injector to activate may result in patients not receiving the required dose of adrenaline resulting in the worsening of symptoms of anaphylaxis or anaphylactic reactions, which could be life threatening."
The company says consumers who have a recalled EpiPen batch number should replace it with a new one as soon as possible by returning it to their pharmacist. They say it will be replaced free of charge.
Officials advise consumers to keep affected auto-injectors until they are replaced and use it if required.
Officials say at this time, EpiPen® Jr 150µg Adrenaline Injection Syringe Auto-Injectors and all other batches of EpiPen® 300µg Adrenaline Injection Syringe Auto-Injectors are unaffected and are not subject to the recall.