Theranos CEO, In Philadelphia To Appeal To Scientific Community, Tries To Stop The Bleeding
PHILADELPHIA (CBS) -- It's been a bruising journey for Theranos, the Silicon Valley blood-testing startup once valued at $9 billion. The technology it promoted as a game-changer has been pummeled by reports of inaccuracy, and the government says one of its labs put patient health in jeopardy.
On Monday, its CEO came to Philadelphia to try to turn the page on the controversy, with a presentation to an audience of skeptical scientists at the Pennsylvania Convention Center.
(Dr. Patricia Jones, AACC president): "Are you going to let your devices be tested in other labs?"
(Elizabeth Holmes, Theranos CEO): "We're working on it right now."
More than 2000 members of the American Association for Clinical Chemistry packed the ballroom, expecting to hear Elizabeth Holmes present -- for the first time -- the science behind what Theranos called a breakthrough: the ability to run dozens of tests from only a finger-prick of blood.
"We have a lot of work to do to engage with this community," Holmes admitted.
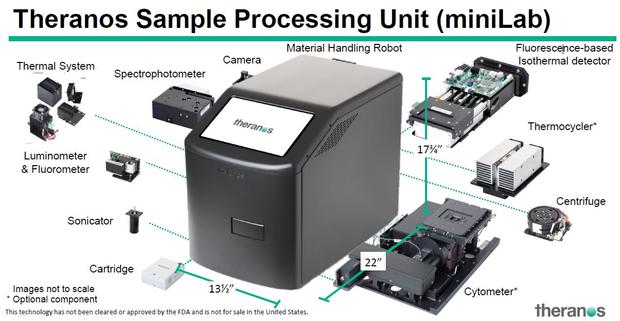
But instead, Holmes introduced a new product: the miniLab, a robotic tabletop testing tool that aims to negate the need for an entire blood lab. She says the device has shown success detecting the Zika virus; Theranos has asked the FDA -- which hasn't cleared or approved miniLab -- for Emergency Use Authorization.
"These results demonstrate the miniLab's ability to perform automated, integrated molecular testing comparable to methods that require highly-trained personnel," Holmes said.
Most of the data in her presentation, though, came not from capillary blood but instead was drawn by needle from a vein, leaving many in the scientific community -- if not regulators and investors -- still questioning the technology Theranos has touted.
Holmes has been banned by the Centers for Medicare and Medicaid Services from owning or operating a laboratory. The federal agency also pulled the license of Theranos' blood-testing facility in California.
"There's clearly been a lot of interest in your company," said Stephen Master, an associate professor at Weill Cornell Medical College and a panelist for the question-and-answer session featuring Holmes and other Theranos officials. "I would argue that a lot of that comes from the fact that there were these claims that were made that were very broad early on -- 70 tests, the whole panoply of lab tests from a couple drops of blood, and the evidence you presented fell far short of that. So, how should we think about that?" he asked, to applause from the audience.
"There's been a lot of news about our company, and we chose this meeting today to begin engaging in a scientific exchange about our inventions and our [miniLab] technology," Holmes responded. "I can tell you I wish that I had started earlier in the context of building the scientific and medical board that we've had the privilege to build and now working toward the peer-reviewed publications. And that's not all going to happen in one day and one presentation. It's going to happen over time."