Recall: Anxiety Medicine By New Jersey-Based Pharmaceutical
BRIDGEWATER, N.J. (CBS) — A Bridgewater-based pharmaceutical company is voluntarily recalling anxiety medicine available to wholesalers nationwide.
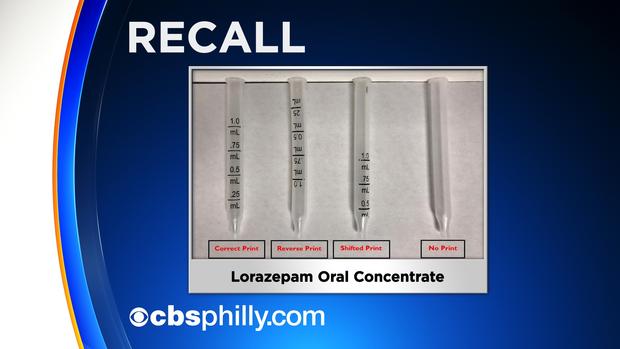
Amneal Pharmaceuticals LLC is recalling 13 lots of Lorazepam Oral Concentrate, USP 2mg/mL, due to a defect in the dropper markings.
According to Amneal Pharmaceuticals, the dropper is printed with the dose markings in reverse number order, has no dose markings or has dose markings that are shifted.
The pharmaceutical company says it learned of the defect via a consumer report and it claims no "adverse events related to these dropper defects have been reported to Amneal."
FDA: Male Enhancement Supplement Recalled Over Hidden Ingredient
The medicine helps with anxiety disorders for the short-term relief of the symptoms of anxiety or anxiety associated with depressive symptoms, according to the manufacturer.
The dropper marking errors could result result in dispensing either less than, or more than, the prescribed dose and this can cause drowsiness, trauma; increased anxiety; increased accidental injury to self or others (i.e. hip fracture, motor vehicle accident); which in the most serious circumstances could result in permanent decreased function or death.
Consumers with questions regarding this recall can contact Amneal Pharmaceuticals at 631-952-0214 (ext. 338) or email: amnealreg@amneal.com Monday through Friday 9 a.m.-5 p.m. ET.
The pharmaceutical company also advises consumers to contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.